Sprayable and Biodegradable, Intrinsically Adhesive Wound Dressing with Antimicrobial Properties
Abstract
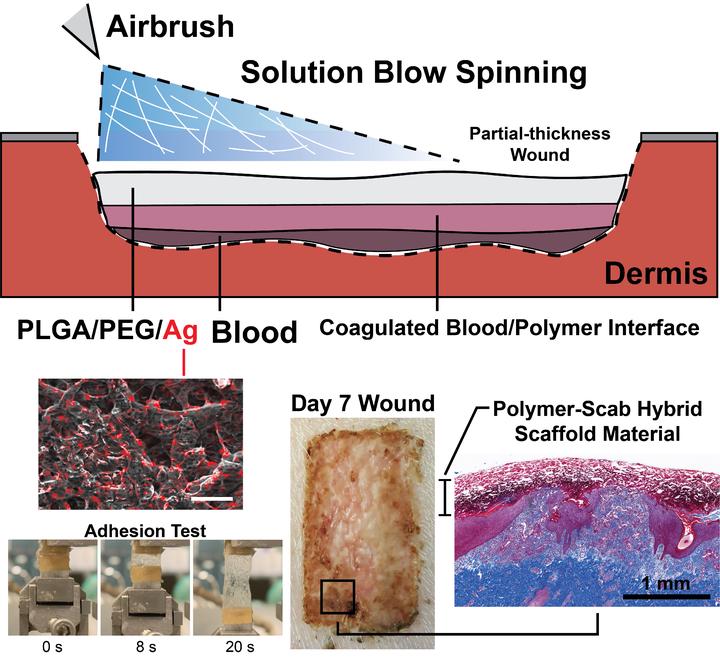
Conventional wound dressings are difficult to apply to large total body surface area (TBSA) wounds, as they typically are prefabricated, require a layer of adhesive coating for fixation, and need frequent replacement for entrapped exudate. Large TBSA wounds as well as orthopedic trauma and low-resource surgery also have a high risk of infection. In this report, a sprayable and intrinsically adhesive wound dressing loaded with antimicrobial silver is investigated that provides personalized fabrication with minimal patient contact. The dressing is composed of adhesive and biodegradable poly(lactic-co-glycolic acid) and poly(ethylene glycol) (PLGA/PEG) blend fibers with or without silver salt (AgNO3). In vitro studies demonstrate that the PLGA/PEG/Ag dressing has antimicrobial properties and low cytotoxicity, with antimicrobial silver controllably released over 7 to 14 days. In a porcine partial-thickness wound model, the wounds treated with both antimicrobial and nonantimicrobial PLGA/PEG dressings heal at rates similar to those of the clinical, thin film polyurethane wound dressing, with similar scarring. However, PLGA/PEG adds a number of features beneficial for wound healing: greater exudate absorption, integration into the wound, a 25% reduction in dressing changes, and tissue regeneration with greater vascularization. There is also modest improvement in epidermis thickness compared to the control wound dressing.