Improving the Adhesion, Flexibility, and Hemostatic Efficacy of a Sprayable Polymer Blend Surgical Sealant by Incorporating Silica Particles
Abstract
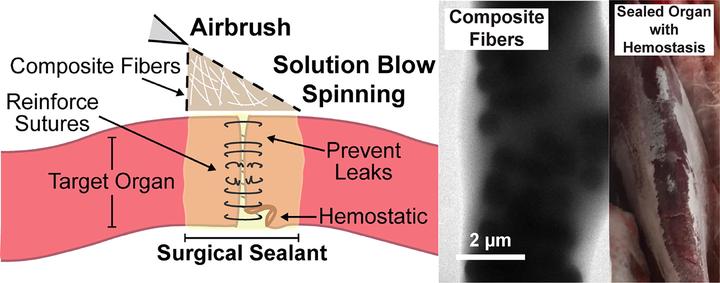
Commercially available surgical sealants for internal use either lack sufficient adhesion or produce cytotoxicity. This work describes a surgical sealant based on a polymer blend of poly(lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG) that increases wet tissue adherence by incorporation of nano-tomicroscale silica particles, without significantly affecting cell viability, biodegradation rate, or local inflammation. In functional studies, PLGA/PEG/silica composite sealants produce intestinal burst pressures that are comparable to cyanoacrylate glue (160 mmHg), ~2 times greater than the noncomposite sealant (59 mmHg), and ~3 times greater than fibrin glue (49 mmHg). The addition of silica to PLGA/PEG is compatible with a sprayable in situ deposition method called solution blow spinning and decreases coagulation time in vitro and in vivo. These improvements are biocompatible and cause minimal additional inflammation, demonstrating the potential of a simple composite design to increase adhesion to wet tissue through physical, noncovalent mechanisms and enable use in procedures requiring simultaneous occlusion and hemostasis.